Chapter XI: Maneesh Dhauni Periodic table- Lithium chemistry
Lithium:
Chemistry is subject of electron reaction. Electron copy proton structural configuration and reaction properties. As seen in the picture, I have shown bonding and anti bonding energy level of Proton and that of Hydrogen and helium. The boding orbital energy level is close to Helium and hence 2 proton acts like Helium in more percentage. The anti bonding energy level is close to Hydrogen and hence it acts like Hydrogen in relatively more percentage.
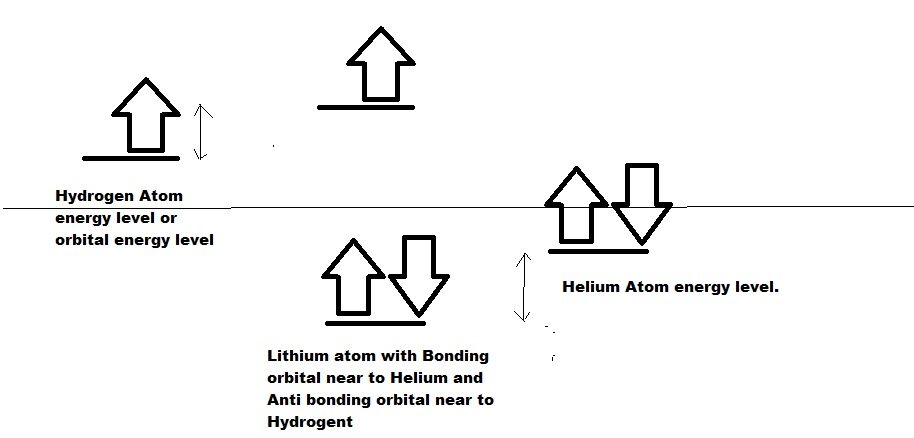
Similar to Protons, electrons also behave the same for Lithium. The 1s orbital of lithium behaves same as that of Helium and 2s orbital of lithium behaves like Hydrogen 1S orbital. Hence, 1s with 2 electrons behave like helium in Lithium and are stable. 2s one electron behave like hydrogen 1s electron and unstable and ready to share with other. Since after sharing, lithium anti bonding becomes empty, it is utilised for coordination bond formation.
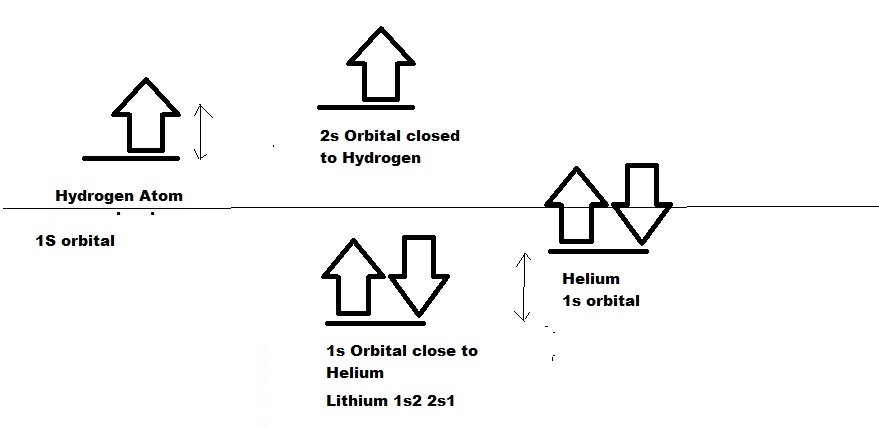
Lithium can be said has He (2) H(1).
Hence we can see lithium behaving like electropositive like Hydrogen and not like other alkali metal.
For lithium monovalent reaction, you can check internet.
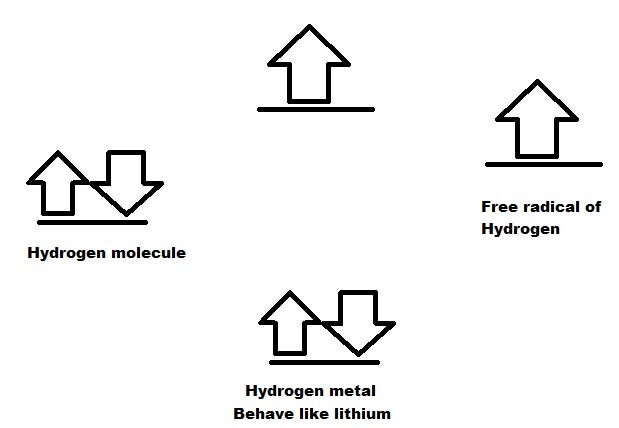
Hydrogen Metal formation:
Hydrogen molecule is stable with 2 electrons as Helium. When hydrogen is treated with very high pressure or Lithium hydride, Hydrogen molecule acts like Helium and react with Hydrogen to form bond and generate Lithium like molecule. This Hydrogen metal can also be generated by treatment of Hydrogen 1 mole with half mole of Helium ion or alpha particles. These are super conductor as there is no resistance.
Maneesh Dhauni periodic table gives better understanding of known and unknown facts of elements and hence it is future of Chemistry.
|
Chemistry facts |
Modern periodic table |
Maneesh Dhauni periodic table |
|
Why Lithium is electropositive with only 1 electron and not 3 electron? |
Fails to explain |
Yes Explains. |
|
Why two electron of 1s orbital are stable? |
Fails to explain |
Yes explains. |
|
Why lithium form co-ordination bond? |
Fails to explain |
Yes explain. |
|
Why hydrogen form Hydrogen Metal and acts like lithium? |
Fails to explain |
Yes explain. |