Maneesh Dhauni Periodic table Chapter X: Lithium
Lithium is 3rd element. Modern periodic table fail to explain following
- Rare abundance of Lithium in Earth crust.
- Greater abundance in sea compare to Land.
- Have an unexpectedly low nuclear binding energy per nucleon.
- Why only Li-6 and Li-7 isotopes occur in nature.
- Hydrogen act like metal under certain condition – especially Lithium.
Other characteristic of lithium is explained by both Modern periodic table and Maneesh Dhauni periodic table.
Lithium is formed by two fusion reactions:
- Hydrogen isotopes and Helium isotopes.
- Between Helium isotopes.
Formation of different isotopes of Lithium is tabulated as follows:
|
Isotopes fusion |
Helium-2 isotope Unstable |
Helium-3 Isotope |
Helium-4 isotope |
Helium-5 isotope Unstable |
Helium-6 isotope Unstable |
|
Hydrogen |
Lithium-3 isotope |
Lithium-4 isotope |
Lithium-5 isotope |
Lithium-6 isotope |
Lithium-7 isotope |
|
Deuterium |
Lithium-4 isotope |
Lithium-5 isotope |
Lithium-6 isotope |
Lithium-7 isotope |
Lithium-8 isotope |
|
Tritium |
Lithium-5 isotope |
Lithium-6 isotope |
Lithium-7 isotope |
Lithium-8 isotope |
Lithium-9 isotope |
|
Maneesh Dhauni remark. |
Since Helium-2 isotope having very short life, these lithium isotope from Helium -2 isotope is very rare. |
As Helium-3 isotope is stable, so we shall discuss formation of these lithium isotope using nuclear orbital. |
As Helium-4 isotope is stable, so we shall discuss formation of these lithium isotope using nuclear orbital. |
Since Helium-5 isotope having very short life, these lithium isotope from Helium -5 isotope is very rare. |
Since Helium-6 isotope having very short life, these lithium isotope from Helium -6 isotope is very rare. |
Ration of Lithium isotope formation is as follow
1 [Lithium-4] : 2[Lithium-5] : 2 [Lithium-6] : 1 [Lithium-7]
In nature, Deuterium and Tritium are rare say less than 1 percentage. So, concentration of Lithium 6 and Lithium 7 is very less. Also Lithium 4 and lithium 5 are radioisotopes. This overall factor explains very less abundance of Lithium on Earth.
Also, as abundance of Hydrogen, deuterium and tritium is more in ocean, concentration of Lithium is more in ocean than in land.
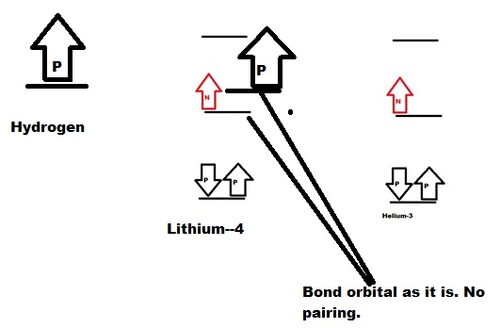
Lithium-4 isotope:
This isotope is formed in nature due to fusion of Hydrogen with Helium-3 isotope.
As given below, Lithium-4 contain Boding orbital with two protons. Non bonding orbital with neutron and proton.
Bonding orbital show property of Helium and stable. Non bonding orbital proton show property of Hydrogen. Due to cluster of charges, Lithium-4 isotope is unstable and decay.
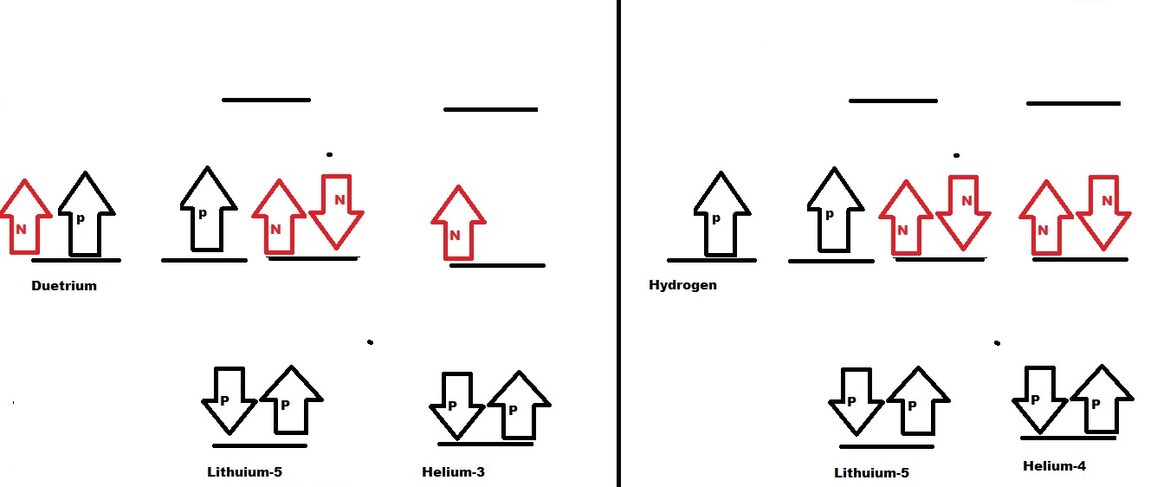
Lithium-5 isotope:
Lithium 5 isotope is formed by fusion of
- Hydrogen and Helium-3 isotope.
- Deuterium and Helium-4 isotope.
Lithium-5 isotope contain one bonding orbital with two protons. Two nonbonding orbital with one electron and two neutrons. Antibonding orbital is empty. Due to overcrowding, Lithium-5 isotope is unstable and decay.
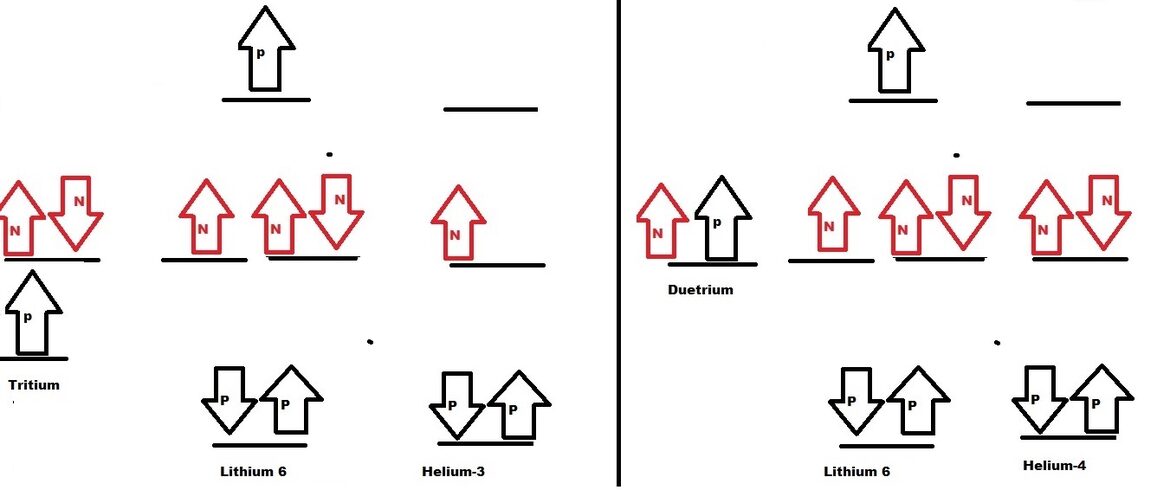
Lithium-6 isotope:
Lithium 6 isotope is formed by fusion of
- Deuterium and Helium-4 isotope.
- Tritium and Helium-3 isotope.
Lithium-6 isotope contain one bonding orbital with two protons. Two nonbonding orbital three neutrons. Antibonding orbital is occupied by Proton, Lithium-6 have tendency to decompose on less energy supply. Hence Lithium-6 have comparatively less binding energy.
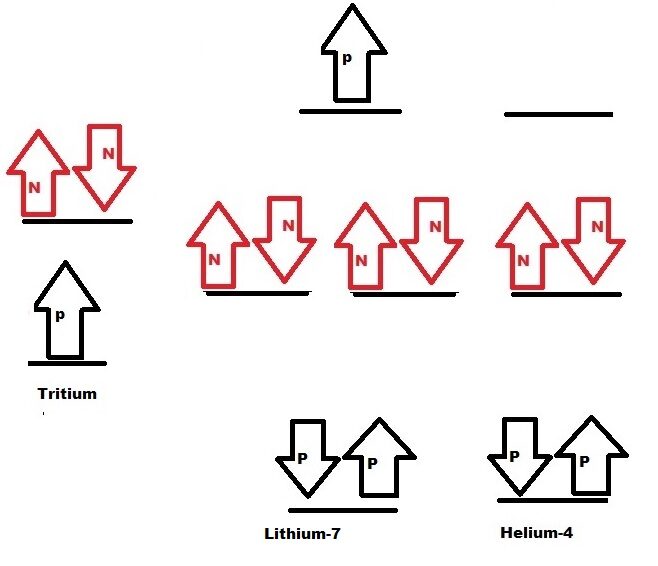
Lithium-7 isotope:
Lithium 7 isotope is formed by fusion of
- Tritium and Helium-4 isotope.
Lithium-7 isotope contain one bonding orbital with two protons. Two nonbonding orbital is occupied by 4 neutron. Antibonding orbital is occupied by Proton, Lithium-7 have tendency to decompose on less energy supply. Hence Lithium-7 have comparatively less binding energy.
Abundance of Lithium-7 is greater than Lithium-6.
As per Maneesh Dhauni periodic table, abundance of Lithium-6 should be greater than Lithium-7. However in nature, it is reported that Lithium-7 occurred more than Lithium 6. Also it is stated that ocean is having larger abundance of than in Land. So it is possible in error in calculation of Human as ocean is not studied completely. After accounting ocean, overall concentration of Lithium 6 should be greater than Lihtium-7.
As Helium is stable element at Nucleus, it is difficult to reacts with Hydrogen isotopes. Hence, formation of Lithium is difficult in one side. Also due to low binding energy, Lithium is unstable and further participate in nuclear fusion to give more elements. Hence considering all above, very less abundance of Lithium is evidence.
Super conductor:
As Lithium is formed due to fusion of Helium and Hydrogen. Similar to nuclear reaction, there is imitation in electronic form. Hydrogen molecule react with Hydrogen radical to form Lithium like metal bond and is conduct electricity. This explains Metallic character of Hydrogen under certain condition. This Hydrogen in lithium like form, may even act like super conductor.
Hydrogen Molecule + Hydrogen radical= n [Hydrogen] where n[ Hydrogen] have lithium like property and also act like super conductor.
This super conductor or metal character of Hydrogen is not explained by Modern Periodic table.
Summary of the above.
|
Chemistry factor |
Modern periodic table |
Maneesh Dhauni periodic table |
|
Fail to explain. |
Yes, explained correctly. |
|
Fail to explain. |
Yes, explained correctly. |
|
Fail to explain. |
Yes, explained correctly. |
|
Fail to explain. |
Yes, explained correctly. |
|
Fail to explain. |
Yes, explained correctly. |
From the above, it is confirm that Maneesh Dhauni Periodic table explain chemistry factor clearly and future of the chemistry.